Neuralink, Elon Musk’s brain-machine interface (BMI) company, has announced that it has received approval from the US Food and Drug Administration (FDA) to conduct its first tests on humans. The company is developing minimally invasive brain chips which it hopes to use to restore vision and mobility for people with disabilities.
Neuralink says it doesn’t have immediate plans to recruit participants, however the FDA approval marks a significant step forward after a previous bid was rejected on safety grounds.
In March, Reuters reported the FDA’s major safety concerns involved the device’s lithium battery, the potential for the implant’s tiny wires to migrate to other areas of the brain, and questions over whether and how the device can be removed without damaging brain tissue.
Musk’s BMI startup first revealed a wireless version of its ‘N1 Link’ implant working in pigs in 2020, which streamed neural data in order to track limb movement. It has since showcased its neural implants working in primates, notably allowing a macaque test subject to play Pong using only its thoughts.

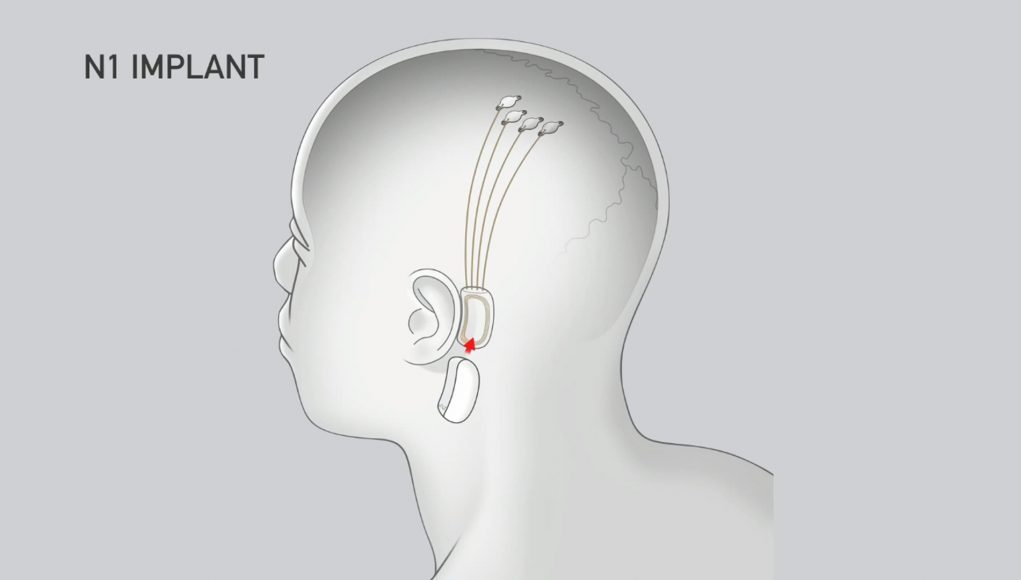
Neuralink’s N1 Implant is hermetically sealed in a biocompatible enclosure which the company says is capable of withstanding harsh physiological conditions. The N1 Implant is implanted by a custom a surgical robot; Neuralink says this ensures accurate and efficient placement of its 64 flexible threads which are distrusted to 1,024 electrodes.
Powered by a small lithium battery that can be wirelessly charged using a compact, inductive charger, the implant is said to incorporate custom low-power chips and electronics that process neural signals and transmit them wirelessly to the Neuralink Application.
Neuralink is currently focused on giving people with quadriplegia the ability to control computers and mobile devices with their thoughts. In the future, the company hopes to restore capabilities such as vision, motor function, and speech, and eventually expand “how we experience the world,” the company says on its website.
That last bit is undoubtedly the company’s most ambitious goal, which the company has said will not only include reading electrical brain signals from paralyzed and neurotypical users alike, but also eventually the ability to “write” signals back to the brain.